Author: Boat Accessories Australia Date Posted: 16 August 2017
The words electrolysis and galvanic corrosion can send a shiver through even the most hardened skipper. Damage can vary from a blister on the paintwork to large metal boat parts being eaten away. The harsh environment means it’s an inevitable part of boating, but there are a few things you can do to help reduce the risks and minimise any potential damage.

Rust is the most well-known form of corrosion. Most metals in manufactured products want to return to their natural state and that is why over time rust can look similar to the brown-red powder of mined iron ore. An example of this would be the brownish flakes that appear on a steel or iron fence that is exposed to external factors like sun and rain.
Oxidation is another form of corrosion that affects only certain metals where oxygen causes the atoms or compounds of a metal to lose its electrons, resulting in an oxide film which will appear differently depending upon the type of metal. Luckily as the oxide layer grows, the rate of electron transfer decreases so some purer metals will remain safe. That being said, the oxidation process may continue if the electrons succeed in entering the metal through cracks or impurities in the metal.
Electrolysis occurs when an electrical current strays from its path due to improper wiring or a defect coming between two metals in the presence of an electrolyte, usually seawater in this case. Metal hull boats are particularly at risk because the hull is conductive and stray wire or connections use the hull as a ground. The two metals can be the same or different.
Galvanic corrosion is when two different metals are in contact in the presence of an electrolyte. One metal will be more chemically active than the other, and a reaction occurs. Very pure water will not conduct electricity, so the electrolyte isn’t present. Saltwater however conducts an electrical current and allows for corrosion to occur.
Metals can be ranked from cathode (negatively-charged electrodes) to anode (positively-charged electrodes). Platinum, titanium, alloy and stainless steel are at the top of the table and considered cathodes, copper nickel, nickel silver, stainless steel (400 series) sit around the middle of the table and depending on the corresponding metal can be either the cathode or anode. At the bottom of the table, and most likely to be the anode, include aluminium alloys, zinc and magnesium.
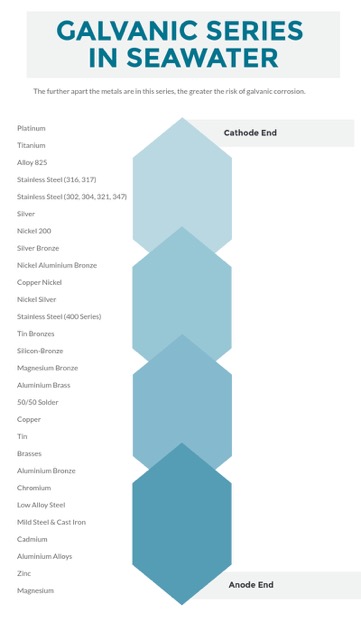
Electrons flow from the more chemically active metal (anode) via a conductive path (seawater) to the less chemically active metal (cathode).
Sacrificial anodes made from aluminium alloys, zinc or magnesium will usually be eaten away quickly when reacting with a metal at the top of the table such as platinum or titanium.
Unfortunately seawater is an efficient electrolyte due to its chloride content and so the added presence of oxygen makes the attack even more effective. As discussed previously, corrosion-resistant metals use an oxide film to ward off the corrosion.
There are five ways to protect your boat against corrosion including:
With regular maintenance and vigilance, you can slow the rates of electrolysis, corrosion and rust on your boat.
Whether it’s stored on land or in the water, anodes will offer your boat the greatest protection from corrosion. Being the sacrificial metal and saving the more expensive metals on your boat is the role of the anode. To ensure the effectiveness of your anodes, keep them in good condition and never paint them.
Obviously, you are reducing your boat’s risks by removing it from the water when not in use, but don’t be lulled into a false sense of security that your boat is on land. All metals have the potential to corrode; it’s just how quickly. A galvanised trailer is protected until the zinc coating is damaged or worn away, leaving the underlying steel exposed. Wash down your trailer after each use to prevent corrosive salt penetrating the coating. If you see any patches of rust, try to clean and sand back the area it’s located before coating with a galvanised spray.
You can protect the metals on your boat or trailer using a rust prevention coating like the Bel-Ray Rust Preventative Coating that seals the metal in similar fashion to what happens in the oxidation process.
Always use similarly charged materials. For example, if you have an aluminium fitting, use aluminium fasteners like screws and rivets. If you used stainless steel screws, you could cause a volatile combination attaching a hard metal against a soft one.
If your boat is left at a marina it’s at more risk, particularly if there are several boats on the same arm. When boats are made of different metals and penned side by side, they will create a current. Faulty wiring can leak current so vessels berthed nearby are at high risk of electrolysis.
Boat owners should leave their extension cords at home and only use marine certified leads that have sufficient insulation around the cable. Coupling the plugs is important to ensure there is no current escaping. To stop any magnetic fields, turn off all power when you not on board or at least reduce the draw of electrical devices. Remember the more current, the more potential for corrosion. A strong current can eat away metals very quickly.
Isolate batteries when not in use and keep all cell components clean as dirt can facilitate a current leak. Make sure you pick up any metal items and don’t leave them sitting at the bottom of your hull. A stray hook or sinker looks pretty harmless but it could cause significant damage to your hull.
A hull reference test can be conducted by taking off all anodes and placing a probe next to the boat’s hull to determine the ambient voltage. Add anodes to the water to determine how many kilos of anodes are needed to mount on the hull. Fit the anodes to your vessel and make regular checks on them as they are your first defence against electrolysis and galvanic corrosion. For more information on hull reference tests, contact your local marina. In the case of a possible electrolysis problem on board, a marine electrician may be needed to test, locate and fix the faults.
Considering the damage galvanic corrosion, electrolysis and oxidation can do to your boat’s parts and hull, it’s worth taking the time to make sure your boat is adequately protected.
Boat Electrolysis
By: Linton Farnham on 22 April 2020Hi there
I have a plate alloy boat with electrolysis problem
Is there some sort of professional you can identify where the problem lays
Location Gold Coast
Thanks
Linton
Boat Accessories Australia Response
Hi Linton,
You should be able to find a local marine electrician who is experienced with fixing electrolysis issues.
Try googling 'marine electrician gold coast'.
Let us know if you have any more questions, and good luck.
Cheers
Rachel